Paint solvents are substances, typically in a liquid state, which form a solution when combined with solid substances possessing similar chemical properties. That is, "like dissolves like".
A solvent for a solid substance has to possess enough kinetic energy to permit cleavage of the molecular structure of that solid. Dissolution of a solid is a process of disassociation of whole molecules with one another. The molecules of a solvent must be able to interact with the molecules of a solute in order for the disassociation to occur. In simple terms, most solids become part of a solution because their individual molecules are pried-apart or simply cut-loose from one another. Once a solution is formed, individual molecules of the solvent and solute are in suspension, free to roam about.
In most cases, the solution looses energy in the form of heat as it is formed and it is cooler than the starting temperature of the solvent as a result. Adding energy in the form of heat, or through the agitation of the solvent and solute, speeds up the process of forming a solution.
Another way to classify solvents is to refer to their molecular polarity, i.e. as being polar or non-polar. Polar solvents possess a net negative charge on one side of their molecules and a net positive charge on the opposite side. Non-polar solvents do not have a polar orientation and are "charge-neutral". One of the simplest examples of a polar solvent is water. Alcohols, esters, ketones and glycol ethers are all polar solvents while the hydrocarbon fractions and terpenes are non-polar.
The solvents used in paints are usually removed entirely from the coating through evaporation as part of the film formation (drying) process. In some exception cases, a solvent like oil for the resins of a varnish can also become part of the dry film, or can be retained in the dry film as with ethanol retention in shellac coatings.
The evaporation process is critical with paints to make the transition from an easy to apply substance to a dry film. Unfortunately the solvents are then free to move about in the atmosphere. Revisions of the Clean Air Act of 1970 have stipulated limits and reduction schedules for the use of VOCs (Volatile Organic Compounds) and HAPs (Hazardous Air Pollutants) in coatings. These government imposed limits and reduction schedules for various classes of coatings have required paint formulators to sometimes make dramatic product changes.
A key solvent parameter is the evaporation rate. The evaporation rate of a solvent is a relative, unit-less, numeric value that typically ranges from 0.001 to 10.0. In the coatings industry the assigned numeric value for evaporation rate is< made relative to n-Butyl Acetate, which therefore sets its evaporation rate at 1.0. So on data sheets you may see the evaporation rate expressed as a number with a notation that (BuAc=1.0).
It should be noted that the real evaporation rate of a solvent can be influenced by something called vapor pressure. If a solvent is miscible (can be readily combined) with water, the evaporation rate of this solvent is reduced as humidity increases. Solvent vapors heavier than air can also form a barrier that inhibits evaporation regardless of the humidity level. Air movement across the surfaces being coated enhances the evaporation process.
The evaporation rate is one of the most important aspects of selecting a solvent for a paint formulation or by a practitioner that merely wants to reduce (thin) the viscosity of a coating or wishes to slow (retard) the film formation process.
A true solvent for a paint resin is one which simply dissolves the resin at room temperature and places it into solution. In lacquer formulations for example, multiple solvents are used but usually only 1 or 2 true solvents are present for the resins in the lacquer.
Latent solvents are included in lacquer formulations to control the formation of the dry lacquer film, usually with spray application in-mind. Latent solvents can also serve as co-solvents with true solvents. Sometimes it is possible to lower the quantity of a more expensive true solvent by selecting a latent solvent that is synergistic with the true solvent for a lacquer resin.
Some of the same considerations for choosing latent solvents should be used when selecting retarding solvents to slow drying in high temperature conditions.
Diluents are solvents that are added to lower the cost of a lacquer formulation and to lower the viscosity of the lacquer to a usable level. You can think of diluents as compatible, but relatively inexpensive reducers or thinners.
Many of the organic solvents used in the paint industry fall into 3 main categories according to their chemical structure; hydrocarbon solvents, oxygenated solvents and halogenated solvents. Other types of solvent families exist like the Terpenes which includes turpentine and D-limonene. Water of course is an important solvent as well.
Hydrocarbon solvents are derived from the refinement of crude oil or coal tar. Each of the solvents listed below can be obtained by heating crude oil (aliphatics) or coal tar (aromatics) to a temperature range that allows each solvent to vaporize. This allows the collection of the solvent through a condensation process by simply passing the vapors through a zone of lower temperature. The process is referred to as fractional distillation.
Aliphatics
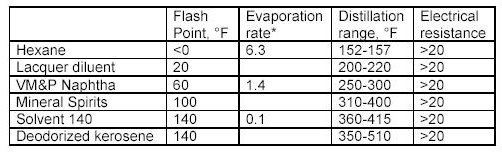
* The most common standard for expressing the evaporation rate
of a solvent is with respect
to the evaporation rate of n-Butyl Acetate which has been assigned a
value of 1.0.
Aromatics
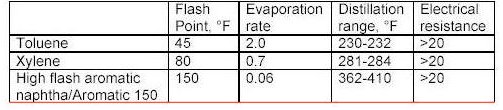
Oxygenated solvents contain oxygen atoms in their composition. This class of solvents is produced by various chemical reactions involving 2 or more components called chemical intermediates rather than simple distillation. Oxygenated solvents possess a broad array of physical properties and are prevalent in paint formulations today.
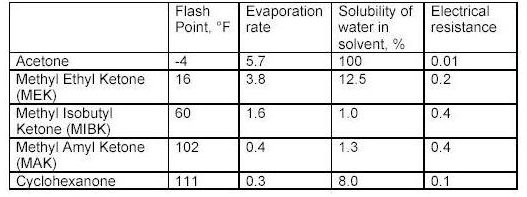
Ketones
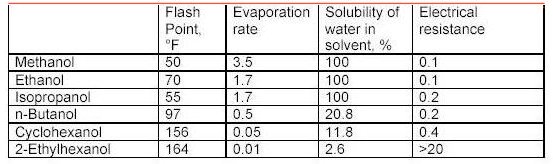
Alcohols
Esters
Glycol Ethers
Glycol Ether Esters (Acetates)
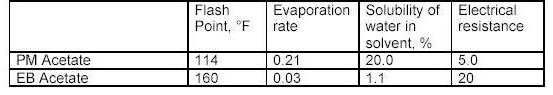
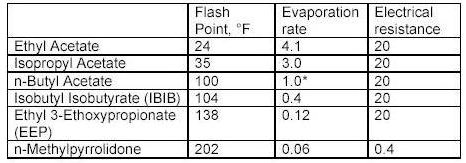
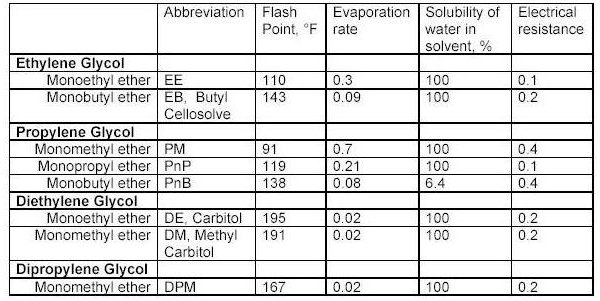
What the tables above tell us?
Flash point is a measurement of how much heat is required to cause a solvent to< freely ignite. Usually this value for a solvent is obtained using a precision instrument called a closed cup test chamber with a heating element. The chamber is warmed to produce enough solvent vapors to cause ignition. Flash point is important in that many wood coatings are considered hazardous materials due to the flash point of the solvents in the formulations. Solvents with flash points less than 100F are considered flammable whereas those with flash points greater than 100F are considered combustible. Special shipping considerations have to be taken into account due to flash point and these are outlined in 49 CFR by the US Department of Transportation.
As discussed above, evaporation rate is an important solvent parameter. The numbers above provide an indication of how fast a solvent will flash-off the surface of a wet paint film. For a user of paints, the values above provide some indication of each solvents behavior as a paint film dries. The time it takes each type of paint to dry on the average is greatly influenced by the solvents that dominate the formulation by volume. Solvents that evaporate slower than these dominant solvents can be considered for use as retarders. Solvents with a relative fast evaporation rate can be useful in cooler conditions or anytime rapid surface drying is desired.
Solvent blush can be attributed to the ability of the solvents present to adsorb or repel water. High humidity levels and the temperature reduction that occurs on a wet paint surface, as a result of evaporation and application of the coating, will allow condensed water (since the wet film surface is much cooler than the room air) to be adsorbed into the hydrophilic (water loving) solvents present in the spray-applied coating. The affinity for water that a solvent has should be a consideration when selecting reducers or retarding solvents. Butyl Cellosolve for example not only mixes readily with water, it also attracts it. Methyl Amyl Ketone on the other-hand is not water-loving and tends not to enhance the effects of high humidity.
As it turns out, the electrical resistance of solvent blends in wet paint is quite an important factor. The resins, and any pigments or clays, used in paint formulation are electrical insulators for the most part - i.e. they do not conduct electricity very well. This is a handy property for a dry paint film for many reasons, especially over metal surfaces. However, when paint is applied and still wet the opposite is true in general. A basic fact of physics sheds some light on why this is the case. An electric charge applied to the surface of a conductor quickly spreads out very evenly. A charge applied to an insulator stays put, roughly where it was applied.
Electric charge distribution has quite a lot to do with molecular behavior and with surface tension in general. Energy can neither be created nor destroyed; it just moves around a lot and changes from one form to another. Electric charges are always present around us. We are all familiar with electric charge in the form of what we call static electricity. In the absence of any other forces or sources of energy, electric fields are responsible for the influence of resin molecules in wet paint films.
The key influence that electric charge has over wet paint films is with respect to surface tension. Simply put, surface tension increases as the density of charge on a surface increases. Many surfaces that paint is applied to are insulators possessing relatively high Critical Surface Tensions. The way to think of Critical Surface Tension is that it is a measure of the average amount of force it will inherently exert to repel a fluid coming into contact with it. Paint will not wet a surface and flow-out evenly if the surface tension of the wet paint material is below that of the Critical Surface Tension of the surface being painted.
The Critical Surface Tension of a wooden surface for example is easy to overcome. However, for any given spot on the surface the Critical Surface Tension varies a bit. Sharp edges and abrupt changes in the surface profile (open grain pockets) create high electric field densities, and therefore hot spots in the Critical Surface Tension, at these edges. This is the science behind why wet paint defects such as craters or fat edges occur. Additives called surfactants (surface active agents) work to help increase the surface tension (or force exerted by the wet edge) of the paint product.
When the solvent blend in a paint product produces a wet film with a low resistance, it helps to evenly distribute the electric field over the surface of the paint. Once the electric field spreads out in a more uniform fashion, the surface and the wet paint film reach equilibrium and uniform coverage of the surface is achieved with fewer defects as a result. The information in the tables above can serve as a guide to those solvents which may make sense to add to avoid or problem.
Copyright 2003 Russ Ramirez
